Accredited Healthcare Software Developers By
Our Medical Device Software Development Services
-
Custom Software for Medical DevicesWe create software tailored to your device’s function diagnostics, monitoring, imaging, or therapy, ensuring smooth performance and accurate data handling.
-
AI & ML IntegrationTrust our medical device software development company to add AI for smart diagnostics, anomaly detection, and predictive insights that enhance decision-making and improve patient care.
-
Connectivity & IoMT IntegrationEnable real-time data sync with IoMT devices, Bluetooth tech, and standards like HL7 and FHIR for seamless healthcare connectivity.
-
Cross-Platform Cloud SolutionsWe build secure, cloud-native apps accessible across mobile, web, and desktop for clinicians and patients anytime, anywhere.
-
Legacy System ModernizationAs part of our medical device app development services, we upgrade outdated software with modern UI, scalable architecture, and integrated AI while ensuring regulatory compliance.
-
Regulatory Compliance & SecurityWe follow FDA, HIPAA, and IEC 62304 standards, using encryption, audit trails, and secure access controls for full compliance.
We're AI-Powered Medical Device Software Development Company
Modern healthcare demands connected and intelligent devices that deliver real-time insights. At Citrusbug Technolabs, we blend domain expertise with cutting-edge technology to develop medical device software that is secure, adaptive, and future-ready.
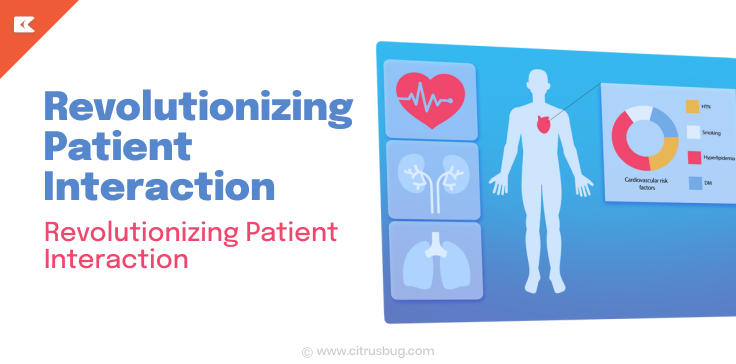
Our solutions go beyond basic functionality by integrating AI, real-time monitoring, and decision support tools. From image recognition in radiology devices to continuous vitals tracking in wearables, we build software that improves medical device workflows and patient outcomes.
We also enable data visualization dashboards, custom analytics modules, and integration with EMR/EHR systems to streamline operations and support healthcare delivery.

Are you searching for best medical device software development companies in India & USA?
Connect with our experienced medical device app developers for expert guidance and tailored solutions that perfectly align with your business goals.
Developing Different Models of Medical Device Software
Remote Patient Monitoring
Enable continuous health tracking with real-time alerts. Aggregate vitals and health data from wearables or in-home devices for better chronic care and post-surgery monitoring.
Diagnostic Imaging Tools
Develop software that supports DICOM, PACS, and AI-based scan analysis. Help radiologists and specialists detect anomalies faster and with greater precision.
Smart Therapy Devices
Power devices that assist in treatments like insulin pumps, neuromodulation, or respiratory therapy. Automate control settings based on patient vitals and clinical rules.
Data Analytics & Reporting
Enable clinicians to view longitudinal data, generate detailed reports, and receive predictive alerts. Custom dashboards help improve care decisions and regulatory readiness.
Device Management Systems
Allow manufacturers and providers to manage device updates, firmware patches, performance logs, and user permissions remotely through secure cloud platforms.
Clinical & Regulatory Integration
Connect software with hospital systems (EHRs/EMRs), labs, and regulatory data workflows. Ensure easy documentation, version control, and compliance audit readiness.
How Much Does it Cost to Develop Medical Device Software?
The cost to develop medical device software typically ranges from $40,000 to $500,000+, depending on complexity, compliance needs, and features.
Every project is unique—share your requirements to get an accurate estimate tailored to your device.
Compliance & Certifications of Medical Device Software We Develop
We design and develop software that meets global healthcare regulatory and safety standards. Our approach ensures compliance, reduces risk, and accelerates market readiness.
FDA 21 CFR Part 820 / 11
Compliance with U.S. regulatory standards for quality systems and electronic records used in medical devices.
HIPAA
Ensures data protection for software that processes or stores patient information, especially in monitoring and diagnostic applications.
IEC 62304
Software development lifecycle compliance for medical devices to meet international safety and risk management standards
ISO 13485 & ISO 27001
Supports secure and quality-assured software development processes tailored for healthcare and medical device companies.
Secure APIs & Data Encryption
All integrations and data transfers use end-to-end encryption and OAuth 2.0 protocols to protect PHI and ensure access control.
Our Medical Device Software Development Process

We begin by immersing ourselves in your vision, your device’s purpose, and your regulatory landscape. Through detailed research & stakeholder discussions, we map out a precise, strategic plan tailored for medical device software.
Designing software for medical devices is more than aesthetics—it's about usability, safety, and empathy. Our team crafts interfaces that feel intuitive to clinicians and patients alike, balancing innovation with the strict usability standards required in healthcare environments.

When it’s time to build, we combine domain expertise with modern engineering. Using secure, FDA/ISO-compliant technologies and agile development, we create scalable and high-performing software—whether it's for diagnostics, monitoring, or connected care.

We don’t just ship software—we deliver peace of mind. By iterating through rigorous testing cycles, refining based on real-world feedback, and aligning with regulatory milestones, we ensure your medical software is safe, reliable, and ready to make a real difference in patients' lives.

Why Citrusbug is Your Ideal Medical Software Development Agency
Our medical device software development company is rated 4.7 on clutch with 30+ client reviews, and we’re a top rated plus agency on Upwork. We proudly maintain a 97% job success rate and have successfully delivered 500+ projects, showcasing our commitment to quality, reliability, and client satisfaction.

Transparency & Integrity
We uphold the highest standards of transparency and integrity, protecting your medical device software ideas through strict NDAs and secure development practices.

On-Time Delivery
Our team follows a structured, milestone-driven approach to ensure your medical device software is delivered on schedule, without compromising on quality or compliance.

Cost-Efficient
We provide high-quality medical device software development services at competitive rates, balancing cost-efficiency with innovation, security, and long-term scalability.

Vast Technical Knowledge
Our developers bring deep domain expertise in healthcare and AI development, enabling us to build advanced, compliant, and future-ready digital health solutions.

QA and Testing
Each medical device software undergoes rigorous testing to ensure a secure, bug-free, and fully functional product that’s ready for clinical and patient use.

24x7 Availability
Our support team remains accessible around the clock to address queries, updates, or concerns via chat, email, or call, ensuring smooth collaboration at every stage.
Client Testimonials (We're Rated 4.7 on Clutch)
Some Top-Notch Healthcare Software Projects We Developed
Advinow is an AI-driven healthcare platform that automates patient engagement and consultation processes, helping healthcare providers deliver efficient, on-demand services while improving operations for urgent care.
Carepoint is a solution dedicated to the pharmacy industry with a variety of tools needed to manage any pharmacy. This pharmacy management solution also offers integral support to manage dispensing, medication therapy, compounding support, A/R & reconciliation, and perpetual inventory.
Furthermore, this tool provides the ability to manage different virtual or real inventories with minimum effort. This platform also offers a range of customization for flexibility for any individual pharmacy. Users can make the most out of this tool and resolve any of the challenges that they generally face.
Droice Labs is a middleware designed to transform messy, unstructured patient data into clean, analysis-ready formats for clinical trials. Ensuring HIPAA/GDPR compliance, the platform automates data processing from EHRs, labs, and claims, providing scalable and high-quality regulatory-compliant insights.

Phelix is a no-code, virtual assistant designed for healthcare workflows by automating patient communication, scheduling, and payment processes. With advanced AI features like OCR, generative AI, chatbot functionality, and SMS automation, Phelix enhances efficiency, reduces administrative burden, and ensures good patient experiences. Phelix supports over 31 million patients across Canada and helps healthcare providers to focus on delivering quality care.
FAQs on Medical Device Software Development
We develop software for Class I, II, and III medical devices, including diagnostics, monitoring systems, and wearables. Our solutions are tailored to suit both embedded systems and standalone applications.
The timeline varies based on complexity, class of device, and regulatory requirements. Typically, development takes 4 to 12 months, including design, testing, validation, and documentation stages.
Absolutely. We specialize in building interoperable software that integrates with EHRs, HIS, and other hospital systems. This ensures seamless data exchange, improved workflow efficiency, and better patient outcomes.
We follow a robust SDLC tailored for medical devices, starting with regulatory scoping, risk and usability engineering, secure DevOps pipelines, iterative prototyping, strict validation/verification, and full documentation to meet IEC/FDA/ISO standards.
We provide comprehensive documentation to support regulatory submissions including Design History Files (DHF), Software Requirements Specifications (SRS), and Risk Management Reports. Our experts can also assist with FDA 510(k) and CE Mark processes.




 SaaS Development
SaaS Development Web Application Development
Web Application Development Mobile Application Development
Mobile Application Development Custom Software Development
Custom Software Development Cloud Development
Cloud Development DevOps Development
DevOps Development MVP Development
MVP Development Digital Product Development
Digital Product Development Hire Chatbot Developers
Hire Chatbot Developers Hire Python Developers
Hire Python Developers Hire Django Developers
Hire Django Developers Hire ReactJS Developers
Hire ReactJS Developers Hire AngularJS Developers
Hire AngularJS Developers Hire VueJS Developers
Hire VueJS Developers Hire Full Stack Developers
Hire Full Stack Developers Hire Back End Developers
Hire Back End Developers Hire Front End Developers
Hire Front End Developers AI Healthcare Software Development & Consulting
AI Healthcare Software Development & Consulting Healthcare App Development
Healthcare App Development EHR Software Development
EHR Software Development Healthcare AI Chatbot Development
Healthcare AI Chatbot Development Telemedicine App Development Company
Telemedicine App Development Company Medical Billing Software Development
Medical Billing Software Development Fitness App Development
Fitness App Development RPM Software Development
RPM Software Development Medicine Delivery App Development
Medicine Delivery App Development Medical Device Software Development
Medical Device Software Development Patient Engagement Software Solutions
Patient Engagement Software Solutions Mental Health App Development
Mental Health App Development Healthcare IT Consulting
Healthcare IT Consulting Healthcare CRM Software Development
Healthcare CRM Software Development Healthcare IT Managed Services
Healthcare IT Managed Services Healthcare Software Testing services
Healthcare Software Testing services Medical Practice Management Software
Medical Practice Management Software Outsourcing Healthcare IT Services
Outsourcing Healthcare IT Services IoT Solutions for Healthcare
IoT Solutions for Healthcare Medical Image Analysis Software Development Services
Medical Image Analysis Software Development Services Lending Software Development Services
Lending Software Development Services Payment Gateway Software Development
Payment Gateway Software Development Accounting Software Development
Accounting Software Development AI-Driven Banking App Development
AI-Driven Banking App Development Insurance Software Development
Insurance Software Development Finance Software Development
Finance Software Development Loan Management Software Development
Loan Management Software Development Decentralized Finance Development Services
Decentralized Finance Development Services eWallet App Development
eWallet App Development Payment App Development
Payment App Development Money Transfer App Development
Money Transfer App Development Mortgage Software Development
Mortgage Software Development Insurance Fraud Detection Software Development
Insurance Fraud Detection Software Development Wealth Management Software Development
Wealth Management Software Development Cryptocurrency Exchange Platform Development
Cryptocurrency Exchange Platform Development Neobank App Development
Neobank App Development Stock Trading App Development
Stock Trading App Development AML software Development
AML software Development Web3 Wallet Development
Web3 Wallet Development Robo-Advisor App Development
Robo-Advisor App Development Supply Chain Management Software Development
Supply Chain Management Software Development Fleet Management Software Development
Fleet Management Software Development Warehouse Management Software Development
Warehouse Management Software Development LMS Development
LMS Development Education App Development
Education App Development Inventory Management Software Development
Inventory Management Software Development Property Management Software Development
Property Management Software Development Real Estate CRM Software Development
Real Estate CRM Software Development Real Estate Document Management Software
Real Estate Document Management Software Construction App Development
Construction App Development Construction ERP Software Development
Construction ERP Software Development




















 BOOK A 30 MIN CALL
BOOK A 30 MIN CALL